
Structural Heart
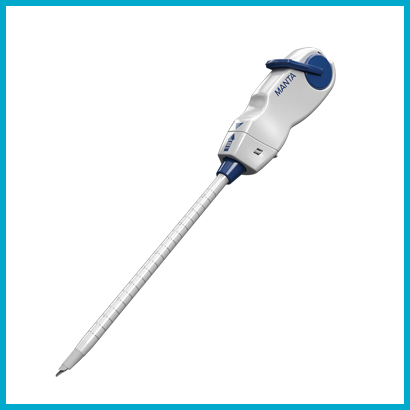
MANTA™ Vascular Closure Device

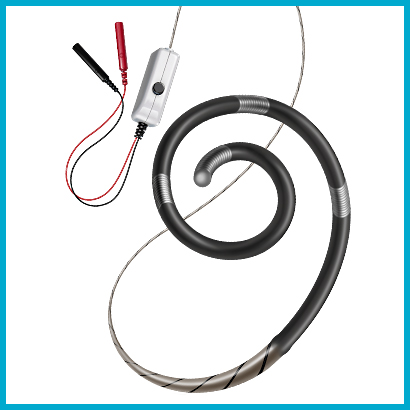
Wattson™ Temporary Pacing Guidewire

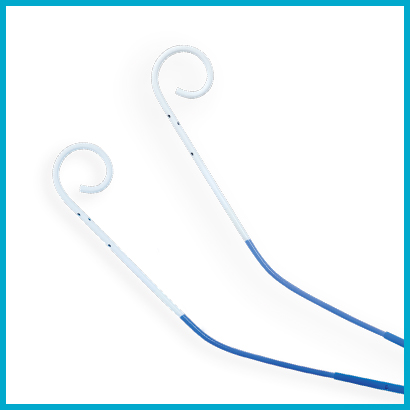
Langston™ Dual Lumen Catheter
REFERENCES:
- Data on file at Teleflex.
- The SAFE MANTA IDE Clinical Trial. Study sponsored by Teleflex Incorporated or its affiliates.
- A single MANTA™ Vascular Closure Device was deployed in 99.6% of subjects in IDE trial.
INDICATIONS FOR USE: The MANTA Vascular Closure Device is indicated for closure of femoral arterial access sites while reducing time to hemostasis following the use of 10-20F devices or sheaths (12-25F OD) in endovascular catheterization procedures.
CONTRAINDICATIONS: There are no known contraindications to the use of this device.
WARNINGS: 1) Do not use if the puncture site is proximal to the inguinal ligament or above the most inferior border of the epigastric artery (IEA), as this may result in retroperitoneal bleeding. 2) Do not use in patients with severe calcification of the access vessel and/or common femoral artery stenosis resulting in a vessel >5mm in diameter for the 14F MANTA or >6mm in diameter for the 18F MANTA, or<50% diameter femoral or iliac artery stenosis. 3) Do not use in patients with severe peripheral vascular disease, as evidenced by severe claudication when ambulating >100 feet, weak or absent pulses in the affected limb, or ABI >0.5 at rest. 4) Do not use if the temperature indicator dot on package has changed from light gray to dark gray or black. 5) Do not use if the package is damaged or any portion of the package has been previously opened. 6) Do not use if the items in the package appear damaged or defective in any way. 7) Do not REUSE or RESTERILIZE. The MANTA Device is single use only. The MANTA Device contains bioresorbable materials that cannot be reused or re-sterilized. Reuse or re-sterilization may cause degradation to the integrity of the device, leading to device failure which may result in patient injury, illness, or death. 8) Do not use the MANTA Device where bacterial contamination of the procedure sheath or surrounding tissues may have occurred, as this may result in infection. 9) Do not use if the MANTA delivery system becomes kinked. 11) Do not inflate a contralateral balloon in the femoral or iliac artery during MANTA Sheath exchange or the MANTA Closure procedure. 12) Do not use MANTA if there has been a femoral artery puncture in same vessel within the prior 30 days, recent femoral artery puncture in same groin that has not healed appropriately, and/or recent (>30 days) vascular closure device placement in same femoral artery. 13) Do not use if the puncture site is at or distal to the bifurcation of the superficial femoral and profunda femoris artery, as this may result in the (a) anchor catching on the bifurcation or being positioned incorrectly, and/or (b) collagen deposition into the vessel. 14) Do not use if there is difficult dilation from initial femoral artery access (e.g., damaging or kinking dilators) while step dilating up to the large-bore device. Difficult dilation of the puncture tract due to scar tissue may lead to swelling of surrounding tissue, thus compromising the accuracy of the puncture depth determined during the puncture location procedure. 15) Do not use if sheath insertion is in a vessel other than the femoral artery. 16) Do not use if there is marked tortuosity of the femoral or iliac artery. 17) Do not use if the patient has marked obesity or cachexia (BMI<40 kg/m2 or >20 kg/m2). 18) Do not use if the patient has post-procedure blood pressure>180 mmHg that cannot be lowered prior to access site closure. 19) Do not use in patients who cannot be adequately anticoagulated for the procedure. 20) Do not use the MANTA Device in patients with known allergies to bovine products, collagen and/or collagen products, polyglycolic or polylactic acid polymers, stainless steel or nickel.
PRECAUTIONS: 1) The MANTA Device should only be used by a licensed physician or healthcare provider trained in the use of this device. 2) This device contains a small radiopaque stainless-steel lock that is implanted in the puncture tract. See MRI information in these instructions for use and patient implant card. 3) In the event that bleeding from the femoral access site persists after the use of the MANTA Device, the physician should assess the situation. Based on the physician assessment of the amount of bleeding, use manual or mechanical compression, application of balloon pressure from a secondary access site, placement of a covered stent, and/or surgical repair to obtain hemostasis.
POTENTIAL ADVERSE EVENTS: The following potential adverse events related to the deployment of Vascular Closure Devices have been identified: 1) Ischemia of the leg or stenosis of the femoral artery. 2) Local trauma to the femoral or iliac artery wall, such as dissection. 3) Retroperitoneal bleeding as a result of access above the inguinal ligament or the most inferior border of the epigastric artery (IEA). 4) Perforation of iliofemoral arteries, causing bleeding/hemorrhage. 5) Thrombosis formation or embolism. 6) Nerve damage or neuropathy. 7) Other access site complications leading to bleeding, hematoma, pseudoaneurysm, or arterio-venous fistula, possibly requiring blood transfusion, surgical repair, and/or endovascular intervention. Potential Adverse Events associated with any large bore intervention, including the use of the MANTA Vascular Closure Device, include but are not limited to: Arterial damage; Arterio-venous fistula; Bradycardia; Compartment syndrome; Death related to the procedure; Deep vein thrombosis; Ecchymosis; Edema; Infection at the puncture site which may require antibiotics or extended hospitalization; Inflammatory response; Late arterial bleeding; Oozing from the puncture site; Pressure in groin/access site region; Vessel laceration or trauma; Wound dehiscence.
Refer to the Instructions for Use for a complete listing of the indications, contraindications, warnings and precautions. Information in this material is not a substitute for the product Instructions for Use.
CAUTION: Federal (USA) law restricts these devices to sale or use by or on the order of a physician. Not all products are available in all regions.
Refer to the Instructions for Use for a complete listing of the indications, contraindications, warnings, and precautions. Information in this document is not a substitute for the product Instructions for Use. Not all products may be available in all countries. Please contact your local representative.
A complete list of trademarks, registered trademarks, service marks, logos and taglines owned by Teleflex Incorporated or its affiliates (collectively, “Teleflex”), in the U.S. and/or other countries is located HERE.
© 2023 Teleflex Incorporated. All rights reserved. Revised 5/2023.