
Rapid. Reliable. Simple.2a-c
Close with more confidence.
A device for large bore closures.
The MANTA Vascular Closure Device is the first-of-its-kind tool specifically designed for large bore femoral arterial access site closure following percutaneous transcatheter interventions.1
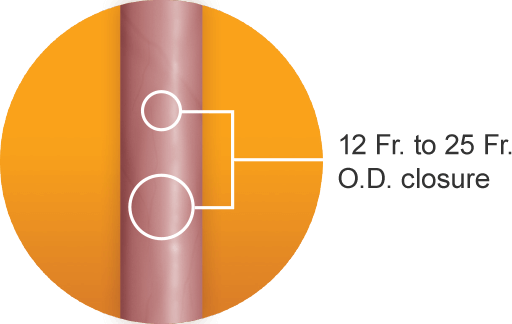
A single MANTA Device is designed to achieve fast, reliable closure with rapid hemostasis in femoral arterial access sites ranging from 12 Fr. to 25 Fr. O.D. with low major complication rates.2a-d
Building Confidence.

Rapid Hemostasis
Efficiently and reliably reduces time to hemostasis without pre-closure.2b-c
- 25.2 second median time for TAVR procedures (73.2 ± 169.8 second mean time)2e
- 19 second median time for EVAR procedures (35 second mean time)4

Easy to use
Proven results with only one device, its straightforward auditory “click” cues make it dependably simple to deploy and easy to use.2a

Low Complication Rates
Provides a low major complication rate of 5.3%2d, and 4.2% VARC-2 Major Vascular Complication Rate (VARC-2 rate lower than published rates for suture-mediated closure)5,6, inspiring confidence in your choice for large bore closure. Every complication avoided can mean additional savings for your health system.

High Technical Success Rate
The device’s effective technology results in high rates of technical success:
- 97.6% technical success rate for TAVR procedures2f
- 98% technical success rate for EVAR procedures2f, 4
Innovation can save lives.
And helps shorten time to discharge.
We know that the potential impact of bleeding complications can result in a higher cost of care per patient, potentially resulting in a 2x increase in hospital length of stay, a 3x increase in mortality, and a 60% increase in healthcare costs.7 When you choose the MANTA Device, its innovative design achieves low major complication rates and rapid time to hemostasis, helping your patients get home faster.8,2b,d
What your peers are saying about the MANTA Device.
“The MANTA Device simplifies large bore arterial access site closure. The device is easy to use, has a short learning curve and results in rapid hemostasis.”
– Dr. David Wood Centre For Heart Valve Innovation, Vancouver, Canada
*This statement reflects the personal experience and opinion of the physician. Dr. Wood, Co-Principal Investigator of the SAFE MANTA IDE Clinical Trial, is a consultant of Teleflex Incorporated or its affiliates.
Why my experience differs from Choice Closure
Lessons learnt from the RCT's on large bore arteriotomy closure.
The Karolinska University Hospital Experience and best practices in 1000 TAVI using MANTA
Vascular Closure Device in EVAR: MANTA Game Changer.
Simple and easy to use.2a
MANTA Device Deployment
Request More Information
Interested in seeing the MANTA Device in action or would you like to speak to one of our team? Submit a request more information form and see how the technology and innovation can create a reliable tool for your team.
- Data on file at Teleflex.
- The SAFE MANTA IDE Clinical Trial.
- A single MANTA® Vascular Closure Device was deployed in 99.6% of subjects in IDE trial.
- The MANTA® Device demonstrated a time to hemostasis (TTH) of 24 seconds median time (65 seconds mean time) from deployment to hemostasis, which is lower than published rates for Perclose ProGlide® where Perclose ProGlide® demonstrated a TTH of 9.8 ± 17 minutes (588 ± 1,020 seconds).3
- 97.7% Technical Success, defined as percutaneous vascular closure obtained with the MANTA® Device without the use of unplanned endovascular or surgical intervention.
- Major Complications defined as composite of: i) vascular injury requiring surgical repair/stent-graft; ii) bleeding requiring transfusion; iii) lower extremity ischemia requiring surgical repair/additional percutaneous intervention; iv) nerve injury (permanent or requiring surgical repair); and v) infection requiring IV antibiotics and/or extended hospitalization.
- Time to Hemostasis defined as: The elapsed time between MANTA® deployment (withdrawal of sheath from artery) and first observed and confirmed arterial hemostasis (no or minimal subcutaneous oozing and the absence of expanding or developing hematoma).
- Technical success defined as: Percutaneous vascular closure obtained with the MANTA® Device without the use of unplanned endovascular or surgical intervention.Study sponsored by Teleflex Incorporated or its affiliates.
- Nelson PR, et al. A multicenter, randomized, controlled trial of totally percutaneous access versus open femoral exposure for endovascular aortic aneurysm repair (the PEVAR trial). J Vasc Surg. 2014 May;59(5):1081-1193.
- Krajcer, Zvonimir, et al. “Pivotal Clinical Study to Evaluate the Safety and Effectiveness of the MANTA Vascular Closure Device During Percutaneous EVAR and TEVAR Procedures.” Journal of Endovascular Therapy, 20 Mar. 2020, p. 152660282091222., doi:10.1177/1526602820912224.
- Généreux P, et al. Vascular complications after transcatheter aortic valve replacement. J Am Coll Cardiol. 2012 Sept 18;60(12):1043-1052.
- Lauten A, et al. Percutaneous left-ventricular support with the Impella 2.5®-assist device in acute cardiogenic shock: results of the Impella-EUROSHOCK-registry. Circ Heart Fail. 2013 Jan;6(1):23-30.
- Redfors et al, Mortality, Length of Stay and Cost Implications of Procedural Bleeding After Percutaneous Intervention Using Large-Bore Catheters, Journal of the American Medical Association (JAMA) Cardiology, Published online March 18, 2017. *Adjusted relative risk
- Megaly M, et al. Outcomes with MANTA Device For Large-Bore Access Closure After Transcatheter Aortic Valve Replacement: A Meta-Analysis. Structural Heart, 13 Aug. 2020, DOI: 10.1080/24748706.2020.1809755.
INDICATIONS FOR USE:The 14F MANTA is indicated for closure of femoral arterial access sites following the use of 10-14F devices or sheaths (maximum OD/profile of 18F), and the 18F MANTA device is indicated for closure of femoral arterial access sites following the use of 15-18F devices or sheaths (maximum OD/profile of 25F).
CONTRAINDICATION: 1) Severe calcification of the access vessel. 2) Severe peripheral
artery disease. 3) Puncture in the origin of the profundal femoral artery,
above the inguinal ligament, or above the most inferior border of the
epigastric artery (IEA). 4) Sheath insertion in vessel other than the femoral
artery. 5) Marked tortuosity of the femoral or iliac artery. 6) Marked obesity
or cachexia (BMI >40 or <20). 7) Blood pressure > 180 mmHg. 8) Patients
who cannot be anti-coagulated for the procedure.
WARNINGS: WARNINGS: 1) Do not use if the temperature indicator dot on package has changed from light gray to dark gray or black. 2) Do not use if the package is damaged or any portion of the package has been previously opened. 3) Do not use if the items in the package appear damaged or defective in any way. 4) Do not REUSE or RESTERILIZE. The MANTA Device is single use only. The MANTA Device contains bioresorbable materials that cannot be reused or re-sterilized. Reuse or re-sterilization may cause degradation to the integrity of the device, leading to device failure which may result in patient injury, illness, or death. 5) Do not use the MANTA Device where bacterial contamination or infection of the target site is suspected. 6) Do not use if there is substantial bleeding around the Depth Locator at the access site during the Depth Location procedure, as this may result in an inaccurate measurement. 7) Do not use if the procedure sheath has been placed through the superficial femoral artery and into the profunda femoris artery, as this may result in collagen deposition into the superficial femoral artery. 8) Do not use if the MANTA delivery system becomes kinked. 9) Do not inflate a contralateral balloon in the femoral or iliac artery during MANTA Sheath exchange or the MANTA Closure procedure. 10) Do not use MANTA if there has been a femoral artery puncture in same vessel within the prior 30 days, recent femoral artery puncture in same groin that has not healed appropriately, and/or recent (<30 days) vascular closure device placement in same femoral artery. 11) Do not use if the puncture site is at or distal to the bifurcation of the superficial femoral and profunda femoris artery, as this may result in the a) anchor catching on the bifurcation or being positioned incorrectly, and/or b) collagen deposition into the vessel. 12) Do not use if the puncture site is proximal to the inguinal ligament or above the most inferior border of the epigastric artery (IEA), as this may result in retroperitoneal bleeding. 13) Difficult dilation of the puncture tract due to scar tissue may lead to swelling of surrounding tissue, thus compromising the accuracy of the puncture depth determined during the depth location procedure.
PRECAUTIONS: 1) The MANTA Device should only be used by a licensed physician or healthcare provider trained in the use of this device. 2) The MANTA Device should not be used in patients with known allergies to bovine products, collagen and/or collagen products, or polyglycolic or polylactic acid polymers. 3) The MANTA Device should not be used in patients with known allergy to stainless steel or nickel. See MRI information in these instructions for use.
ADVERSE EVENTS: 1) Failed hemostasis requiring manual or mechanical compression, application of balloon pressure from a secondary access site, placement of a covered stent or surgical repair. 2) Local trauma to the femoral or iliac artery wall, such as dissection. 3) Retroperitoneal bleeding, and its consequences, as a result of failed closure in the setting of an access above the inguinal ligament or the most inferior border of the epigastric artery (IEA). 4) Perforation of iliofemoral arteries, causing bleeding/hemorrhage. 5) Accidental positioning of some or all of the collagen plug within the femoral artery, leading to ischemia or stenosis. 6) Thrombosis formation or embolism. 7) Adjacent nerve damage or neuropathy. 8) Other access site complications leading to bleeding, hematoma, pseudoaneurysm, etc., possibly requiring blood transfusion, surgical repair, and/or endovascular intervention.
Teleflex, the Teleflex logo, Arrow, Deknatel, Hudson RCI, LMA, MANTA, Pilling, Rüsch, UroLift and Weck are trademarks or registered trademarks of Teleflex Incorporated or its affiliates, in the U.S. and/or other countries. All other trademarks and registered trademarks are property of their respective owners.
Information in this material is not a substitute for the product Instructions for Use. Not all products may be available in all countries.
Please contact your local representative. Revised: 11/2022.
eleflex, the Teleflex logo, Arrow, Deknatel, Hudson RCI, LMA, MANTA, Pilling, Rüsch, UroLift and Weck are trademarks or registered trademarks ofted or its affiliates, in the U.S. and/or other countries. All other trademarks and registered trademarks are property of their respective
owners.
Information in this material is not a substitute for the product Instructions for Use. Not all products may be available in all countries.
MCI-2019-0231-EN . REV A . 07 19 PDF