A device for large bore closures.
The MANTA® Vascular Closure Device is the first-of-its-kind tool specifically designed for large bore femoral arterial access site closure following percutaneous transcatheter interventions.1
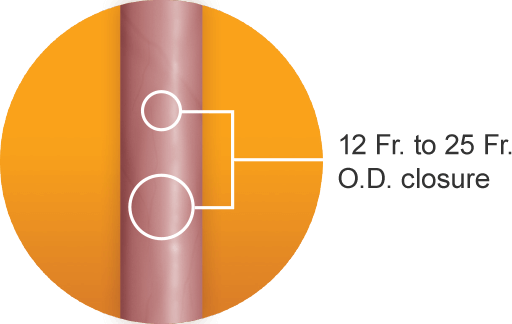
A single MANTA® Device is designed to achieve fast, reliable closure with rapid hemostasis in femoral arterial access sites ranging from 12 Fr. to 25 Fr. O.D. with low major complication rates.2a-d
Building Confidence.

Rapid Hemostasis
Efficiently and reliably reduces time to hemostasis without pre-closure.2b-c
- 25.2 second median time for TAVR procedures (73.2 ± 169.8 second mean time)2e
- 19 second median time for EVAR procedures (35 second mean time)4

Easy to use
Proven results with only one device, its straightforward auditory “click” cues make it dependably simple to deploy and easy to use.2a

Low Complication Rates
Provides a low major complication rate of 5.3%2d, and 4.2% VARC-2 Major Vascular Complication Rate (VARC-2 rate lower than published rates for suture-mediated closure)5,6, inspiring confidence in your choice for large bore closure. Every complication avoided can mean additional savings for your health system.

High Technical Success Rate
The device’s effective technology results in high rates of technical success:
- 97.6% technical success rate for TAVR procedures2f
- 98% technical success rate for EVAR procedures2f, 4
Innovation can save lives.
And helps shorten time to discharge.
We know that the potential impact of bleeding complications can result in a higher cost of care per patient, potentially resulting in a 2x increase in hospital length of stay, a 3x increase in mortality, and a 60% increase in healthcare costs.7 When you choose the MANTA® Device, its innovative design achieves low major complication rates and rapid time to hemostasis, helping your patients get home faster.8,2b,d
Simple and easy to use.2a
MANTA® Device Deployment
- Data on file at Teleflex.
- The SAFE MANTA IDE Clinical Trial.
- A single MANTA® Vascular Closure Device was deployed in 99.6% of subjects in IDE trial.
- The MANTA® Device demonstrated a time to hemostasis (TTH) of 24 seconds median time (65 seconds mean time) from deployment to hemostasis, which is lower than published rates for Perclose ProGlide® where Perclose ProGlide® demonstrated a TTH of 9.8 ± 17 minutes (588 ± 1,020 seconds).3
- 97.7% Technical Success, defined as percutaneous vascular closure obtained with the MANTA® Device without the use of unplanned endovascular or surgical intervention.
- Major Complications defined as composite of: i) vascular injury requiring surgical repair/stent-graft; ii) bleeding requiring transfusion; iii) lower extremity ischemia requiring surgical repair/additional percutaneous intervention; iv) nerve injury (permanent or requiring surgical repair); and v) infection requiring IV antibiotics and/or extended hospitalization.
- Time to Hemostasis defined as: The elapsed time between MANTA® deployment (withdrawal of sheath from artery) and first observed and confirmed arterial hemostasis (no or minimal subcutaneous oozing and the absence of expanding or developing hematoma).
- Technical success defined as: Percutaneous vascular closure obtained with the MANTA® Device without the use of unplanned endovascular or surgical intervention.
Study sponsored by Teleflex Incorporated or its affiliates.
- Nelson PR, et al. A multicenter, randomized, controlled trial of totally percutaneous access versus open femoral exposure for endovascular aortic aneurysm repair (the PEVAR trial). J Vasc Surg. 2014 May;59(5):1081-1193.
- Krajcer, Zvonimir, et al. “Pivotal Clinical Study to Evaluate the Safety and Effectiveness of the MANTA Vascular Closure Device During Percutaneous EVAR and TEVAR Procedures.” Journal of Endovascular Therapy, 20 Mar. 2020, p. 152660282091222., doi:10.1177/1526602820912224.
- Généreux P, et al. Vascular complications after transcatheter aortic valve replacement. J Am Coll Cardiol. 2012 Sept 18;60(12):1043-1052.
- Lauten A, et al. Percutaneous left-ventricular support with the Impella 2.5®-assist device in acute cardiogenic shock: results of the Impella-EUROSHOCK-registry. Circ Heart Fail. 2013 Jan;6(1):23-30.
- Redfors et al, Mortality, Length of Stay and Cost Implications of Procedural Bleeding After Percutaneous Intervention Using Large-Bore Catheters, Journal of the American Medical Association (JAMA) Cardiology, Published online March 18, 2017. *Adjusted relative risk
- Megaly M, et al. Outcomes with MANTA Device For Large-Bore Access Closure After Transcatheter Aortic Valve Replacement: A Meta-Analysis. Structural Heart, 13 Aug. 2020, DOI: 10.1080/24748706.2020.1809755.
