Meet the Arrow™ OnControl™ Powered Bone Marrow Biopsy System.
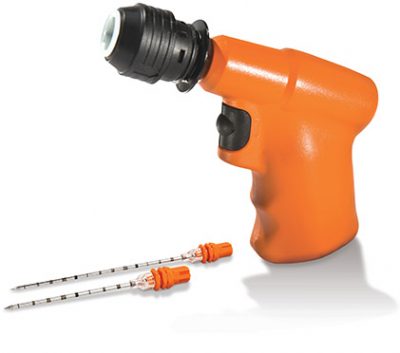
The trusted powered solution for practitioners, pathologists, and patients.
High-quality samples
- As compared to manual needles, the Arrow™ OnControl™ Powered Bone Marrow Biopsy System delivers consistently larger, high-quality core specimens.3-5
- This may reduce the number of second-attempt procedures required3,5-6 and result in more usable area for diagnosis.3,5
Increased user control1,2
Provides precise control7 and rapid access to difficult bone lesions.1
May result in a bone biopsy procedure that is up to 55% faster than with manual needles.3,5
Dependable performance
Specially engineered cannula with unique capture wire secures specimen during extraction.
Comprehensive system trays contain the components needed for high-quality bone lesion biopsies.
Less patient pain
The system has been demonstrated to cause less patient pain—during insertion and after the procedure—as compared to manual needles.3-4
Reduced pain can help ensure patient compliance with ongoing testing.3,6
REFERENCES:
1 Lee RK, Ng AW, Griffith JF. CT-guided bone biopsy with a battery-powered drill system: preliminary results. AJR Am J Roentgenol 2013;201(5):1093-5. doi:10.2214/AJR.12.10521.
2 Symington K, Martinez F, Miller LJ, Philbeck T. Examination of 64 consecutive specimens obtained using a powered biopsy device. JVIR 2014;25(3s):S196. Research sponsored by Teleflex Incorporated.
3 Miller LJ, Philbeck TE, Montez DF, et al. Powered bone marrow biopsy procedures produce larger core specimens, with less pain, in less time than with standard manual devices. Hematology Reports 2011;3:e8.t. Research sponsored by Teleflex Incorporated.
4 Swords RT, Anguita J, Higgins RA, et al. A prospective randomized study of a rotary powered device (OnControl) for bone marrow aspiration and biopsy. J Clin Pathol 2011;64(9):809-13. Research sponsored by Teleflex Incorporated.
5 Reed LJ, Raghupathy R, Strakhan M, et al. The OnControl bone marrow biopsy technique is superior to the standard manual technique for hematologists-in-training: a prospective, randomized comparison. Hematology Reports 2011;3(e21). doi:10.4081/hr.2011.e21. Research sponsored by Teleflex Incorporated.
6 Garcia G, Miller LJ, Philbeck, T, Bolleter S, Montez, D. Tactile feedback allows accurate insertion of a powered bone access device for vertebroplasty and bone marrow sampling procedures. J Vasc and Interv Radiol 2011;22(3):S86. Research sponsored by Teleflex Incorporated.
7 Berenson JR, Yellin O, Blumenstein B, et al. Using a powered bone marrow biopsy system results in shorter procedures, causes less residual pain to adult patients, and yields larger specimens. Diagnostic Pathology 2011;6:23. Research sponsored by Teleflex Incorporated.
The Arrow™ OnControl™ Bone Marrow Aspiration System is intended for bone marrow aspiration of the iliac crest of adult and pediatric patients.
The Arrow™ OnControl™ Bone Marrow Biopsy System is intended for bone marrow core biopsy of the anterior or posterior iliac crest of adult patients.
The Arrow™ OnControl™ Bone Lesion Biopsy System is intended for bone biopsy of the vertebral body and bone lesions.
The Arrow™ OnControl™ Powered Bone Marrow Biopsy System and Powered Bone Lesion Biopsy System should not be used by clinicians unfamiliar with the complications, limitations, indications, and contraindications of bone marrow aspiration and biopsy.
Refer to the Instructions for Use for a complete listing of the indications, contraindications, warnings and precautions. Information in this material is not a substitute for the product Instructions for Use.
Refer to the Instructions for Use for a complete listing of the indications, contraindications, warnings, and precautions. Information in this document is not a substitute for the product Instructions for Use. Not all products may be available in all countries. Please contact your local representative.
A complete list of trademarks, registered trademarks, service marks, logos and taglines owned by Teleflex Incorporated or its affiliates (collectively, “Teleflex”), in the U.S. and/or other countries is located HERE.
© 2023 Teleflex Incorporated. All rights reserved. Revised 5/2023.
